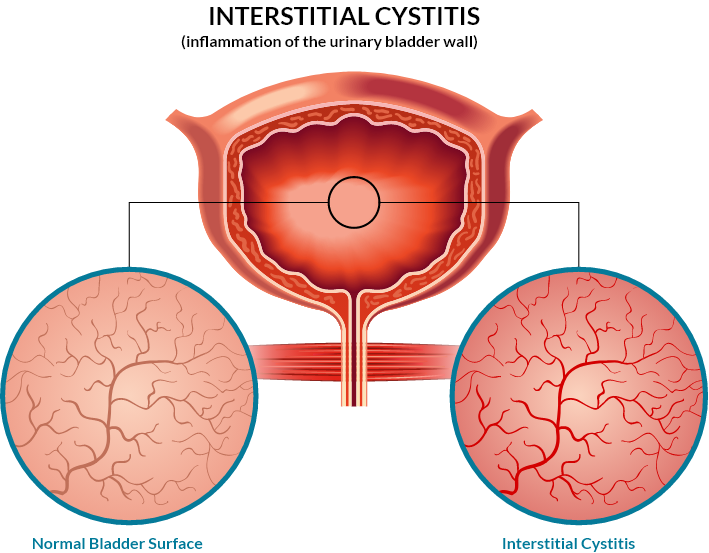
Interstitial Cystitis/Bladder Pain Syndrome (IC/BPS) is a disorder of the bladder. IC/BPS has a debilitating impact on as many as 10M in the US, predominantly women. It is a chronic disease characterized by often debilitating pain, frequent and/or urgent urination and increased associated with bladder filling. The symptoms may range from needing to urinate more often or urgently, to severe pain. Work, daily activities, and social functioning can become very difficult.
Currently there is no cure for IC/BPS. The available interstitial cystitis treatment options do not meet the needs of most patients. Health care providers assist with palliative options to help minimize pain and manage the life-altering symptoms of IC/BPS.
“I cried myself to sleep this morning, I feel depressed, hopeless… I’m on the verge of losing my husband, my family, my life. … I often feel like a huge knife is coming out of me as I pee.” — Becky
“I’ve been searching for answers, but I just feel so hopeless. I want my life back. There are so many things I can’t do. I want to go back to work & need to go back. I am ONLY 24. I don’t know how long I can take living this way.” — Elizabeth
“For over 9 months I have been up 12-14 times at night to use the restroom & this goes on during the day. I am having a difficult time trying to have any type of life.”— Beverly
The exact mechanisms causing IC/BPS remain incompletely understood.
It is known that injury to the internal bladder surface is a common pathogenic feature of the diverse presentations of IC/BPS. This protective surface is made up of glycosaminoglycans (GAGs) which are a chemical class of biopolymers found in soft tissues of the body. This protective layer plays a critical role in maintaining impermeability at the bladder wall. This biopolymer layer has been found to be damaged or missing in many patients suffering from IC/BPS. The breakdown of the impermeable barrier at the luminal bladder wall exposes inner tissues to urine’s noxious substances.
Therefore, a therapy known as “GAG replenishment therapy” was developed by urologists as a treatment option to restore the integrity of the bladder wall.
In bladder wall replenishment therapy, a simple solution of a biopolymer is infused into the injured bladder through a catheter.
The IC/BPS patient holds this solution in the bladder for 15-30 minutes. During this time the biopolymer binds to the bladder wall restoring and repairing the damaged/missing protective layer.
Replenishment is a local therapy with the advantages of delivering high concentrations of biopolymer directly to the bladder wall where it is needed. The biopolymer is non-absorbed, remaining on the bladder wall surface with no systemic absorption that might impact biological processes elsewhere in the body.
Despite the many advantages of a local therapy that directly replenishes the protective layer of the damaged bladder wall, clinical studies have shown that current replenishment products have low to moderate effectiveness.
For that reason, these products have not been approved in the US, leaving clinicians with very limited interstitial cystitis treatment options.
The biopolymers used in currently available replenishment products have been selected simply because they are the only similar materials that are commercially available.
Intentional design of a bladder wall binding biopolymer to have higher affinity and residence time, as Glycologix is now pursuing, has never been attempted.
The potential for a truly potent and efficacious replenishment product remains a viable possibility for the 4-12 million patients suffering with IC/BPS in the US today. Glycologix is developing a biopolymer platform targeting protection of the urothelial tissue. We believe an effective interstitial cystitis treatment could quickly become a new standard of care for those that are suffering.
